ESCHERICHIA COLI STRAIN BL21: CLONING AND EXPRESSION OF AN OPTIMIZED INTERFERON ALPHA 2B (DE3)
Views: 563 https://doi.org/10.59807/jlsar.v1i2.15
https://doi.org/10.59807/jlsar.v1i2.15
Keywords:
Recombinant Protein, Bacterial Expression System, IPTG Induction, Western BlottingAbstract
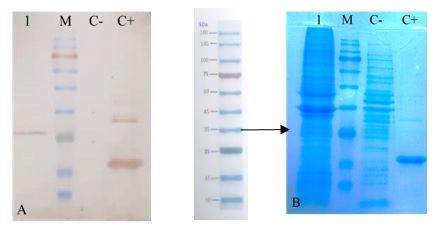
Interferon alpha 2b gene (INF α2b) as a protein with antiviral and antitumor activities is potentially a valuable therapeutic protein to work on. Prior to having a large-scale production of the target protein, it is recommended to examine it on an experimental scale, so that a bacterial host could be a proper choice as it leads us to a deep insight into the subject. In this research, the INF α2b sequence was obtained from the NCBI gene data bank, and after optimization, it was subjected to be cloned and expressed in pET28a+. In order to primary examination of the target protein, Escherichia coli was considered a prokaryotic expression system. IPTG induction of the protein in bacteria cells containing the construct pET: IFN, followed by resolving total proteins through SDS-PAGE. The expected size of the investigated protein, about 24kDa, was observed through gel separation. Further assessment via western blotting confirmed the successful expression of IFN α2b.
Downloads
References
L. Zitvogel, L. Galluzzi, O. Kepp, M. J. Smyth, and G. Kroemer, “Type I interferons in anticancer immunity,” Nature Reviews Immunology, vol. 15, no. 7. 2015. doi: 10.1038/nri3845.
P. J. Hertzog and B. R. G. Williams, “Fine tuning type I interferon responses,” Cytokine and Growth Factor Reviews, vol. 24, no. 3. 2013. doi: 10.1016/j.cytogfr.2013.04.002.
S. Pestka, C. D. Krause, and M. R. Walter, “Interferons, interferon-like cytokines, and their recep-tors,” Immunological Reviews, vol. 202. 2004. doi: 10.1111/j.0105-2896.2004.00204.x.
T. Ariyasu et al., “Effects of interferon-alpha subtypes on the Th1/Th2 balance in peripheral blood mononuclear cells from patients with hepatitis virus infection-associated liver disorders,” In Vitro Cell Dev Biol Anim, vol. 41, no. 1–2, 2005, doi: 10.1290/0501008.1.
P. Lengyel, “Biochemistry of interferons and their actions,” Annu Rev Biochem, vol. Vol.51, 1982, doi: 10.1146/annurev.bi.51.070182.001343.
J. Parkin and B. Cohen, “An overview of the immune system,” Lancet, vol. 357, no. 9270. 2001. doi: 10.1016/S0140-6736(00)04904-7.
R. J. Motzer, J. Bacik, B. A. Murphy, P. Russo, and M. Mazumdar, “Interferon-Alfa as a Compara-tive Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma,” Journal of Clinical Oncology, vol. 20, no. 1, 2002, doi: 10.1200/jco.2002.20.1.289.
T. A. Nyman, N. Kalkkinen, H. Tölö, and J. Helin, “Structural characterisation of N-linked and O-linked oligosaccharides derived from interferon-α2b and interferon-α14c produced by Sen-dai-virus- induced human peripheral blood leukocytes,” Eur J Biochem, vol. 253, no. 2, 1998, doi: 10.1046/j.1432-1327.1998.2530485.x.
P. Srivastava, P. Bhattacharaya, G. Pandey, and K. J. Mukherjee, “Overexpression and purification of recombinant human interferon alpha2b in Escherichia coli,” Protein Expr Purif, vol. 41, no. 2, 2005, doi: 10.1016/j.pep.2004.12.018.
I. Rabhi-Essafi, A. Sadok, N. Khalaf, and D. M. Fathallah, “A strategy for high-level expression of soluble and functional human interferon α as a GST-fusion protein in E.coli,” Protein Engineering, Design and Selection, vol. 20, no. 5, 2007, doi: 10.1093/protein/gzm012.
E. Wood, “Molecular Cloning. A Laboratory Manual,” Biochem Educ, vol. 11, no. 2, 1983, doi: 10.1016/0307-4412(83)90068-7.
W. Zhuo-hua, L. Hong-hao, and M. Hui-wen, “Recovery of DNA from agarose gel with home-made silica milk,” Wuhan University Journal of Natural Sciences, vol. 5, no. 3, 2000, doi: 10.1007/bf02830159.
P. E. Vaillancourt, E. coli Gene Expression Protocols. 2002. doi: 10.1385/1592593011.
W. T. Booth et al., “Impact of an N terminal polyhistidine tag on protein thermal stability,” ACS Omega, vol. 3, no. 1, 2018, doi: 10.1021/acsomega.7b01598.
A. Poetsch, L. L. Molday, and R. S. Molday, “The cGMP-gated Channel and Related Glutamic Ac-id-rich Proteins Interact with Peripherin-2 at the Rim Region of Rod Photoreceptor Disc Mem-branes,” Journal of Biological Chemistry, vol. 276, no. 51, 2001, doi: 10.1074/jbc.M108941200.
P. Stanley, “Golgi glycosylation,” Cold Spring Harb Perspect Biol, vol. 3, no. 4, 2011, doi: 10.1101/cshperspect.a005199.
Y. Guan, Q. Zhu, D. Huang, S. Zhao, L. Jan Lo, and J. Peng, “An equation to estimate the difference between theoretically predicted and SDS PAGE-displayed molecular weights for an acidic pep-tide,” Sci Rep, vol. 5, 2015, doi: 10.1038/srep13370.
R. Radhakrishnan et al., “Zinc mediated dimer of human interferon-α(2b) revealed by X-ray crys-tallography,” Structure, vol. 4, no. 12, 1996, doi: 10.1016/S0969-2126(96)00152-9.
P. A. Arlen et al., “Field production and functional evaluation of chloroplast-derived interfer-on-α2b,” Plant Biotechnol J, vol. 5, no. 4, 2007, doi: 10.1111/j.1467-7652.2007.00258.x.
Published
How to Cite
Issue
Section
Citations
License
Copyright (c) 2020 Creative Commons Attribution 4.0 International (CC-BY 4.0)

This work is licensed under a Creative Commons Attribution 4.0 International License.
This journal is licensed under a Creative Commons Attribution 4.0 International (CC-BY 4.0)