PRODUCTION AND CHARACTERIZATION OF Β-GALACTOSIDASE ENZYME IN THE PLANT EXTRACT FROM (ZIZIPHUS SPINA-CHRISTI) AND ITS APPLICATION IN MILK
Views: 449 https://doi.org/10.59807/jlsar.v2i1.20
https://doi.org/10.59807/jlsar.v2i1.20
Keywords:
Ziziphus spina-christi, β-galactosidase, Enzymatic Kinetics, Heavy MetalsAbstract
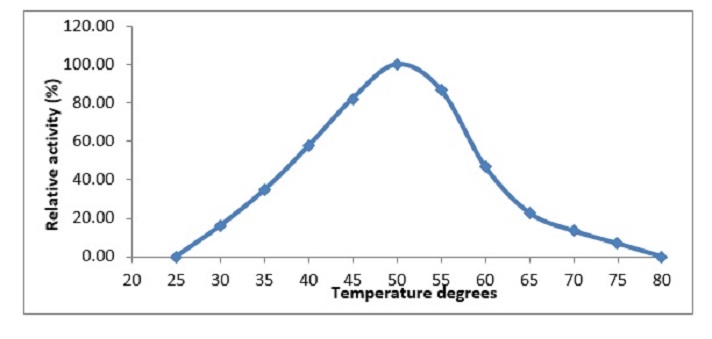
In the development of a medicinal plant, β-galactosidase (EC 3.2.1.23) is essential (Ziziphus spina-christi). The enzyme activity was measured by its ability to hydrolyze the substrate 2-nitrophenyl β-D-galactopyranoside (ONPG). The maximum enzyme activity was at 50 ° C and at pH 5.5. The enzyme's Km and Vmax values were 3.65 mM and 0.18 μmol / min, respectively. HgCl2 and KCN completely inhibit the activities of β-galactosidase (Ziziphus spina-christi). Lactose in milk was reduced by 38.5 and 70 percent by β-galactosidase from (Ziziphus spina-christi), respectively, after 4h incubation. This result showed that the β-galactosidase enzyme in the extract of leaves (Ziziphus spina-christi) can be used for industrial and medical applications.
Downloads
References
B. W. Matthews, “The structure of E. coli β-galactosidase,” Comptes Rendus - Biologies, vol. 328, no. 6 SPEC. ISS. 2005. doi: 10.1016/j.crvi.2005.03.006. DOI: https://doi.org/10.1016/j.crvi.2005.03.006
P. Alliet et al., “Effect of prebiotic galacto-oligosaccharide, long-chain fructo-oligosaccharide infant formula on serum cholesterol and triacylglycerol levels,” Nutrition, vol. 23, no. 10, 2007, doi: 10.1016/j.nut.2007.06.011. DOI: https://doi.org/10.1016/j.nut.2007.06.011
S. W. Przemieniecki, A. Kosewska, S. Ciesielski, and O. Kosewska, “Changes in the gut microbiome and enzymatic profile of Tenebrio molitor larvae biodegrading cellulose, polyethylene and polystyrene waste,” Environmental Pollution, vol. 256, 2020, doi: 10.1016/j.envpol.2019.113265. DOI: https://doi.org/10.1016/j.envpol.2019.113265
A. Pal, M. Lobo Melita, and F. Khanum, “Extraction, purification and thermodynamic characterization of almond (Amygdalus communis) β-galactosidase for the preparation of delactosed milk,” Food Technol Biotechnol, vol. 51, no. 1, 2013.
S. Li, X. Zhu, and M. Xing, “A new β-galactosidase from the antarctic bacterium alteromonas sp. ANT48 and its potential in formation of prebiotic galacto-oligosaccharides,” Mar Drugs, vol. 17, no. 11, 2019, doi: 10.3390/md17110599. DOI: https://doi.org/10.3390/md17110599
F. A. Shaikh, M. Randriantsoa, and S. G. Withers, “Mechanistic analysis of the blood group anti-gen-cleaving endo-β-galactosidase from Clostridium perfringens,” Biochemistry, vol. 48, no. 35, 2009, doi: 10.1021/bi900991h. DOI: https://doi.org/10.1021/bi900991h
A. Jokar and A. Karbassi, “In-house production of lactose-hydrolyzed milk by beta-galactosidase from lactobacillus bulgaricus,” Journal of Agricultural Science and Technology, vol. 13, no. 4, 2011.
F. B. Sudério, G. K. da C. Barbosa, E. Gomes-Filho, and J. Enéas-Filho, “Purification and characterization of cytosolic and cell wall β-galactosidases from Vigna unguiculata stems,” Brazilian Journal of Plant Physiology, vol. 23, no. 1, 2011, doi: 10.1590/S1677-04202011000100003. DOI: https://doi.org/10.1590/S1677-04202011000100003
H. el D. Yossef, “Extraction, Purification and Characterization of Apricot Seed β-Galactosidase for Producing Free Lactose Cheese,” J Nutr Food Sci, vol. 04, no. 02, 2014, doi: 10.4172/2155-9600.1000270. DOI: https://doi.org/10.4172/2155-9600.1000270
D. Kishore and A. M. Kayastha, “A β-galactosidase from chick pea (Cicer arietinum) seeds: Its pu-rification, biochemical properties and industrial applications,” Food Chem, vol. 134, no. 2, 2012, doi: 10.1016/j.foodchem.2012.03.032. DOI: https://doi.org/10.1016/j.foodchem.2012.03.032
A. Saeed, M. Salim, U. Zaman, R. Naz, S. Jan, and A. Saeed, “β-galactosidase from watermelon (Citrullus lanatus) seedlings: Partial purification and properties,” Pakistan Journal of Biotechnology, vol. 14, no. 3, 2017.
S. B. Al-Arrji and N. A. Al-Hamadi, “Extraction, Purification and Characterization of β-Galactosidase from Apricot(Prunus armeniaca kaisa) Fruit for lactose intolerance treatment,” Int J Chemtech Res, vol. 10, no. 6, 2017.
S. Ogasawara, K. Abe, and T. Nakajima, “Pepper β-galactosidase 1 (PBG1) plays a significant role in fruit ripening in bell pepper (Capsicum annuum),” Biosci Biotechnol Biochem, vol. 71, no. 2, 2007, doi: 10.1271/bbb.60179. DOI: https://doi.org/10.1271/bbb.60179
G. H. Dean et al., “The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties,” Plant Cell, vol. 19, no. 12, 2007, doi: 10.1105/tpc.107.050609. DOI: https://doi.org/10.1105/tpc.107.050609
S. Gulzar and S. Amin, “Kinetic Studies on <i>β</i>-Galactosidase Isolated from Apri-cots (<i>Prunus armeniaca kaisa</i>),” Am J Plant Sci, vol. 03, no. 05, 2012, doi: 10.4236/ajps.2012.35077. DOI: https://doi.org/10.4236/ajps.2012.35077
N. Ali, S. Andleeb, B. Mazhar, and A. Khan, “Optimization of β-galactosidase Production from Yogurt and Dairy Soil Associated Bacteria Using Different Fermentation Media,” Br Microbiol Res J, vol. 11, no. 2, 2016, doi: 10.9734/bmrj/2016/18750. DOI: https://doi.org/10.9734/BMRJ/2016/18750
D. P. M. Torres, M. do P. F. Gonçalves, J. A. Teixeira, and L. R. Rodrigues, “Galac-to-Oligosaccharides: Production, properties, applications, and significance as prebiotics,” Compr Rev Food Sci Food Saf, vol. 9, no. 5, 2010, doi: 10.1111/j.1541-4337.2010.00119.x. DOI: https://doi.org/10.1111/j.1541-4337.2010.00119.x
I. Martín, T. Jiménez, R. Esteban, B. Dopico, and E. Labrador, “Immunolocalization of a cell wall β-galactosidase reveals its developmentally regulated expression in Cicer arietinum and its relationship to vascular tissue,” J Plant Growth Regul, vol. 27, no. 2, 2008, doi: 10.1007/s00344-008-9044-9. DOI: https://doi.org/10.1007/s00344-008-9044-9
A. Dwevedi and A. M. Kayastha, “Plant β-galactosidases: Physiological significance and recent advances in technological applications,” Journal of Plant Biochemistry and Biotechnology, vol. 19, no. 1. 2010. doi: 10.1007/bf03323431. DOI: https://doi.org/10.1007/BF03323431
Aldoobie, F. N, Beltagi, and S. M, “Physiological, biochemical and molecular responses of com-mon bean (Phaseolus vulgaris L.) plants to heavy metals stress,” Afr J Biotechnol, vol. 12, no. 29, 2013, doi: 10.5897/ajb2013.12387. DOI: https://doi.org/10.5897/AJB2013.12387
A. Gaur and A. Adholeya, “Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils,” Current Science, vol. 86, no. 4. 2004.
P. Scudder, K. Uemura, J. Dolby, M. N. Fukuda, and T. Feizi, “Isolation and characterization of an endo-beta-galactosidase from Bacteroides fragilis.,” Biochem J, vol. 213, no. 2, 1983, doi: 10.1042/bj2130485. DOI: https://doi.org/10.1042/bj2130485
A. Schützendübel and A. Polle, “Plant responses to abiotic stresses: Heavy metal-induced oxida-tive stress and protection by mycorrhization,” in Journal of Experimental Botany, 2002. doi: 10.1093/jxb/53.372.1351. DOI: https://doi.org/10.1093/jxb/53.372.1351
A. Ramadan et al., “Anti-diabetic effect of Artemisia judaica extracts,” researchgate.net, vol. 4, no. 1, 2009.
I. M. Al-Rawashdeh, “Genetic variability in a medicinal plant Artemisia judaica using random amplified polymorphic DNA (RAPD) markers,” Int J Agric Biol, vol. 13, no. 2, 2011.
Q. Husain, “β Galactosidases and their potential applications: A review,” Critical Reviews in Bio-technology, vol. 30, no. 1. 2010. doi: 10.3109/07388550903330497. DOI: https://doi.org/10.3109/07388550903330497
O. M. Atrooz, “Characterization of β-galactosidase in the Crude Plant Extract of <i>Artemisia judaica</i> L. in Presence and Absence of Some Heavy Metals,” American Journal of Life Sciences, vol. 4, no. 5, 2016, doi: 10.11648/j.ajls.20160405.11. DOI: https://doi.org/10.11648/j.ajls.20160405.11
P. H. N. de Alcântara, L. Martim, C. O. Silva, S. M. C. Dietrich, and M. S. Buckeridge, “Purification of a β-galactosidase from cotyledons of Hymenaea courbaril L. (Leguminosae). Enzyme properties and biological function,” Plant Physiology and Biochemistry, vol. 44, no. 11–12, 2006, doi: 10.1016/j.plaphy.2006.10.007. DOI: https://doi.org/10.1016/j.plaphy.2006.10.007
S. Hussein, M. Imanhashimabdulrazzaq, and S. H. Murtadha, “Extraction and purification of ßeta-galactosidase from a Local Almond seeds(Prunusamygdalus),” IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS, vol. 13, no. 1, 2018.
C. M. S. Carrington and R. Pressey, “β-Galactosidase II activity in relation to changes in cell wall galactosyl composition during tomato ripening,” Journal of the American Society for Horticultural Science, vol. 121, no. 1, 1996, doi: 10.21273/jashs.121.1.132. DOI: https://doi.org/10.21273/JASHS.121.1.132
H. Konno and H. Tsumuki, “Purification of a β‐galactosidase from rice shoots and its involvement in hydrolysis of the natural substrate in cell walls,” Physiol Plant, vol. 89, no. 1, 1993, doi: 10.1111/j.1399-3054.1993.tb01784.x. DOI: https://doi.org/10.1111/j.1399-3054.1993.tb01784.x
S. Shen, X. F. Li, W. R. Cullen, M. Weinfeld, and X. C. Le, “Arsenic binding to proteins,” Chemical Reviews, vol. 113, no. 10. 2013. doi: 10.1021/cr300015c. DOI: https://doi.org/10.1021/cr300015c
O. BARITAUX, M. AMIOT, and J. NICOLAS, “Enzymatic browning of basil (ocimum basilicum L.) studies on phenolic compounds and polyphenol oxidase,” Sciences des aliments, vol. 11, no. 1. 1991.
S. Biswas, A. M. Kayastha, and R. Seckler, “Purification and characterization of a thermostable β-Galactosidase from kidney beans (Phaseolus vulgaris L.) cv. PDR 14,” J Plant Physiol, vol. 160, no. 4, 2003, doi: 10.1078/0176-1617-00748. DOI: https://doi.org/10.1078/0176-1617-00748
R. A. Goyer, “Toxic and essential metal interactions,” Annual Review of Nutrition, vol. 17. 1997. doi: 10.1146/annurev.nutr.17.1.37. DOI: https://doi.org/10.1146/annurev.nutr.17.1.37
D. H. Lee, S. G. Kang, S. G. Suh, and J. K. Byun, “Purification and characterization of a β-galactosidase from peach (Prunus persica),” Mol Cells, vol. 15, no. 1, 2003.
R. A. Harvey, Lippincott’s Illustrated Reviews, Biochemistry, 6th Edition. 2017.
P. K. Jena, D. Trivedi, K. Thakore, H. Chaudhary, S. S. Giri, and S. Seshadri, “Isolation and Characterization of Probiotic Properties of Lactobacilli Isolated from Rat Fecal Microbiota,” Microbiol Immunol, 2013, doi: 10.1111/j.1348-0421.12054. DOI: https://doi.org/10.1111/1348-0421.12054
M. Pagthinathan, H. M. Ghazali, A. M. Yazid, and H. L. Foo, “Extraction, purification and characterization of a milk-clotting protease from ‘kesinai’ (Streblus asper Lour.) leaves,” Int Food Res J, vol. 26, no. 3, 2019.
Published
How to Cite
Issue
Section
Citations
License
Copyright (c) 2022 Journal of Life Science and Applied Research

This work is licensed under a Creative Commons Attribution 4.0 International License.
This journal is licensed under a Creative Commons Attribution 4.0 International (CC-BY 4.0)